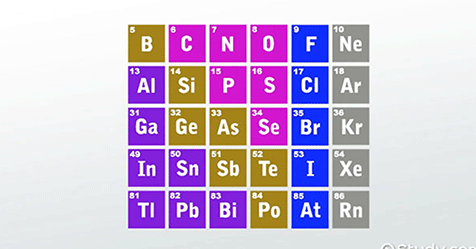
P- Block Elements
Right side group elements of columns 13, 14, 15, 16, 17 and 18 of the periodic table are known as P- Block Elements.


These elements have 3,4,5,6,7 and 8 electrons in their outer most shell respectively and the last electron of these group elements occupies the position in p-subshell that is why they are called p-block elements. Their general configuration is ns2np1.
Properties of P-block:
- Electron affinity: For P-block elements, electron affinity increases from left to right and decreases from top to bottom. But group 15 elements having low values of electron affinity due to extra stability that means the presence of exactly half-filled orbital in their valence shell. Similarly, group 18 have zero electron affinity due to the presence of complete octet which provides stability.
- Metallic Character: The metallic character is defined by the size of atoms and ionization energy.
If the elements have bigger size atoms and low ionization energy then those elements will have a greater metallic character. Based on the combination of above two factors we observe that the elements which have metallic are located in the left corner of P-Block and strong non-metallic are located at the right corner and the diagonal strip of elements separates these two, having in between properties are called as metalloids.
Oxidation state: The P- block elements have a variety of oxidation states both positive and negative. Some elements show different oxidation states due to inert pair effect, where their lower oxidation state is more predominant.
Characteristics of P-block Elements in a Tabular form: