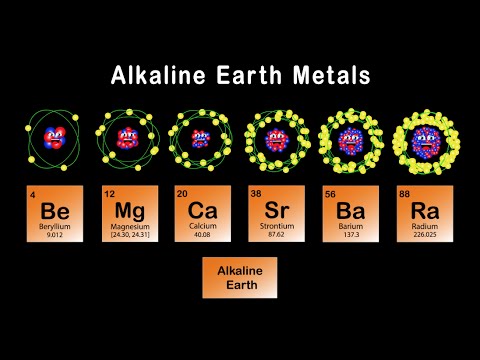
Group 2 – Alkaline Earth Metals
Alkaline earth metals are the second group of the periodic table. This family includes the elements beryllium, magnesium, calcium, strontium, barium, and radium (Be, Mg, Ca, Sr, Ba, and Ra, respectively). Group 2 elements share common characteristics. Each metal is naturally occurring and quite reactive. These metals are silver and soft, much like the alkali metals of Group 1. These metals also react with water, though not as vigorously. Beryllium, interestingly, does not react with water. Each alkaline earth metal has two valence electrons. They will easily give these electrons up to form cations. These metals become increasingly more reactive as you go down the periodic table. This is concurrent with general periodic trends.